www.sciensation.org | Ciênsação hands-on experiments are published as Open Educational resources under a Creative Commons Attribution-ShareAlike 4.0 International License.
www.sciensation.org | Ciênsação hands-on experiments are published as Open Educational resources under a Creative Commons Attribution-ShareAlike 4.0 International License.
Why does wet feel cold?

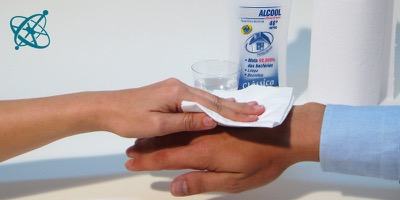
Mix some alcohol with water,…
…to sweep it with a cloth over skin.
Cold breeze
Most students know that they want to go swimming on a hot day, but far less will know why water has such a refreshingly cooling effect. Use this first-hand experience to start a discussion on why evaporation cools, and how mammals, including humans, use sweat to control their body temperature.
Evaporation takes thermal energy from the environment and thus cools it down.
We use this effect to regulate our body temperature.
Water
Pure alcohol (optional)
The cooling effect of evaporation gets stronger if you mix a bit of alcohol with water.
To save time, prepare one wet cloth for each table row and let it pass from student to student.
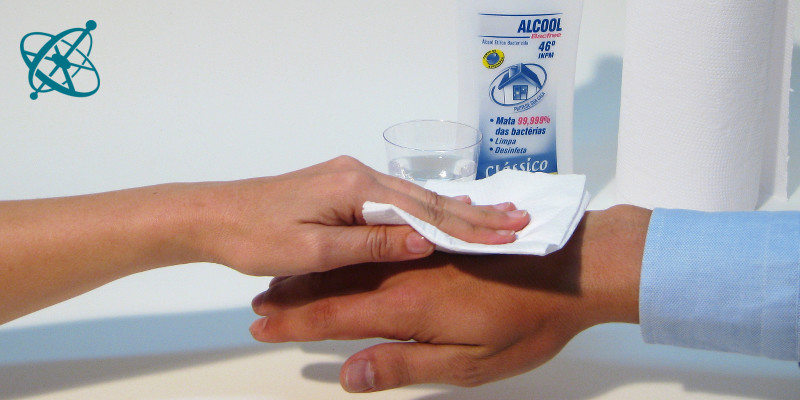
Sweep with a wet cloth over the back of your hand and then blow lightly over it.
1. Why does it feel cooler when air blows over wet skin than when air blows over dry skin?
2. How could you make use of this effect?
Where did the liquid go?
› In the air, it evaporated.
Does it require energy for the liquid to 'take off' into the air, to evaporate?
› Yes.
Where does this energy come from?
› It is taken from the thermal energy of the liquid and the skin, cooling both down.
Instead of simply explaining that evaporation has a cooling effect, help your students to come to this conclusion themselves.
Once the students have understood the concept, you may ask them to explain how their body uses this effect to control its temperature, and what other possible applications they can think of.