 www.sciensation.org | Ciênsação hands-on experiments are published as Open Educational resources under a Creative Commons Attribution-ShareAlike 4.0 International License.
www.sciensation.org | Ciênsação hands-on experiments are published as Open Educational resources under a Creative Commons Attribution-ShareAlike 4.0 International License.
What makes this water rise?

Build a wedge from two microscope slides…
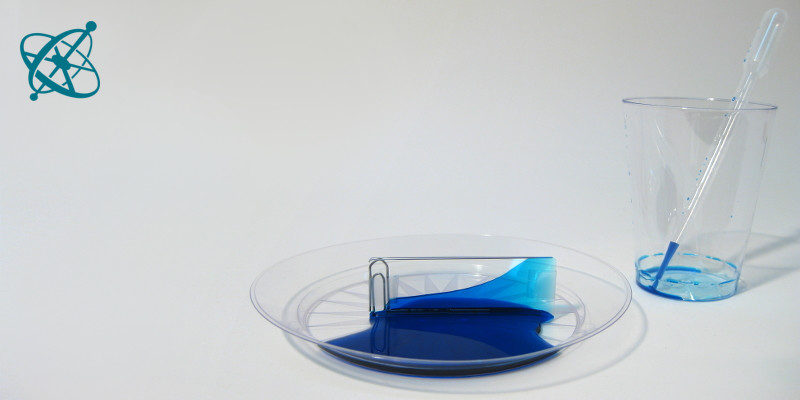
…and place it into the liquid.

Try different liquids!
Capillary forces
A simple yet very effective setup to study capillary forces: Let your students discover for themselves what makes the water rise between two glass slides.
Understanding capillary action.
Developing experiments to study what factors influence an observed effect.
Paper clip (or similar object as spacer)
Sticky tape
Plastic dishes
Test liquids like water, oil, detergent
Food coloring for a nicer effect
While the material is distributed, show your students how to prepare the wedge by taping together two microscope slides with a paper clip as spacer on one side.
Place oil and dishwater liquid well visible on the teacher table where they may 'inspire' your students to use them in additional experiments.
It is recommended to always have some paper towels in reach.
Make the wedge stand in a plastic dish so that the paperclip is upright. Then slowly pour some water into the dish.
1.What factors determine the height to which the liquid is drawn up to?
2. Can you come up with experiments to test your hypothesis?
What changes along the lengths of the two microscope slides?
› The width of the air gap between them.
Does the water rise higher in a smaller or a wider air gap?
› In a smaller gap.
If you look carefully where the air gap is a bit wider: Does the water reach higher in the middle or closer to the glass?
› Closer to the glass.
Do the water molecules seem attracted to the glass?
› Yes.
Does the effect depend on the type of liquid?
› Yes, tests with oil or water mixed with detergent give different results.
The height to which capillary forces can draw the liquid up depends on
1) the size of the air gap between the two glass slides,
2) the surface tension,
3) the liquid's density – due to the gravity's pull, and
4) the molecular (adhesive) forces between the liquid and the surface of the solid (here glass).
While the influence of the gap size is easy to observe, and the effect of the surface tension becomes visible when adding detergent, the last two factors are more difficult to see. The attractive forces between the water and glass can be noted when looking carefully at the upward bending of the water on the glass wall. A comparison of oil and water points to material properties as relevant, but the details will probably require your explanation as teacher.



