 www.sciensation.org | Ciênsação hands-on experiments are published as Open Educational resources under a Creative Commons Attribution-ShareAlike 4.0 International License.
www.sciensation.org | Ciênsação hands-on experiments are published as Open Educational resources under a Creative Commons Attribution-ShareAlike 4.0 International License.
A safe and simple illustration of a catalyst.
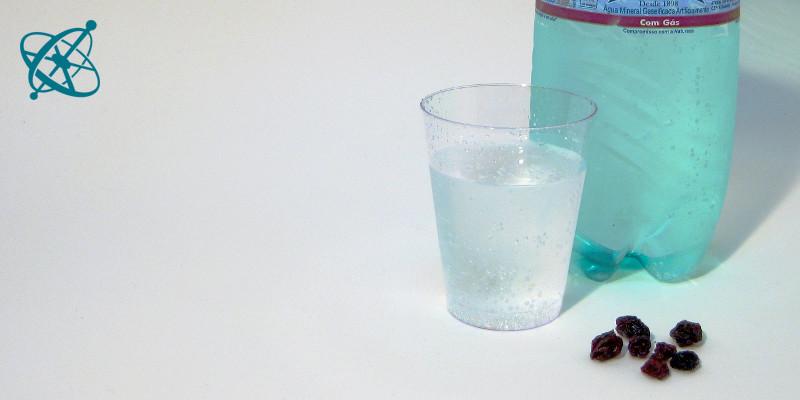
All it takes is sparkling water…

…and some raisins to 'dance' in it.
Raising raisins
Typical catalysis experiments involve fire or rather complex biochemical reactions with enzymes. If you are looking for a safe way to teach the concept hands-on, something just at the right speed to be easily observed, a couple of raisins or rice corns in sparkling water may well do the trick.
A catalyst facilitates or increases the rate of a chemical reaction without being transformed itself.
Sparkling water
Raisins
Put a few raisins into a cup with sparkling water.
1. What makes the raisins raise and sink?
2. What chemical reaction happens on the raisins' surface and what role do the raisins play in this reaction?
3. Are the raisins changed by the chemical reaction?
What are these tiny silvery dots that appear on a raisin when it drops into water?
› Small air bubbles.
Where does the gas come from that makes the water effervesce?
› This is CO2 that was dissolved in the water and now goes back to the gas phase.
In sparkling water, carbon dioxide (CO2) has been added under pressure and cool temperatures to form carbonic acid (H2CO3):
CO2 + H2O ⇌ H2CO3
When the pressure is reduced (e.g. by opening the bottle), or the water's temperature increased, the chemical equilibrium is changed and CO2 raises to the surface as small gas bubbles.
When raisins are put into water, tiny air bubbles remain in the pockets of their rough skin. These air bubbles support the formation of CO2: While in water most of the CO2 quickly dissolves again, the CO2 in air bubbles is far more likely to stay in the gas phase. As the gas accumulates in bubbles on the raisin's skin, it provides buoyancy to the raisin. Lifted to the water surface, some of these bubbles burst, reducing the buoyancy, and so the raisin sinks again.
While the raisin is not transformed by the chemical reaction, it facilitates it with the tiny air bubbles bound to its skin. It thus can be taken as a slow-motion illustration of a catalyst.


