 www.sciensation.org | Ciênsação hands-on experiments are published as Open Educational resources under a Creative Commons Attribution-ShareAlike 4.0 International License.
www.sciensation.org | Ciênsação hands-on experiments are published as Open Educational resources under a Creative Commons Attribution-ShareAlike 4.0 International License.
Can you show that molecules…

…in hot water move faster than…

…in cold water?
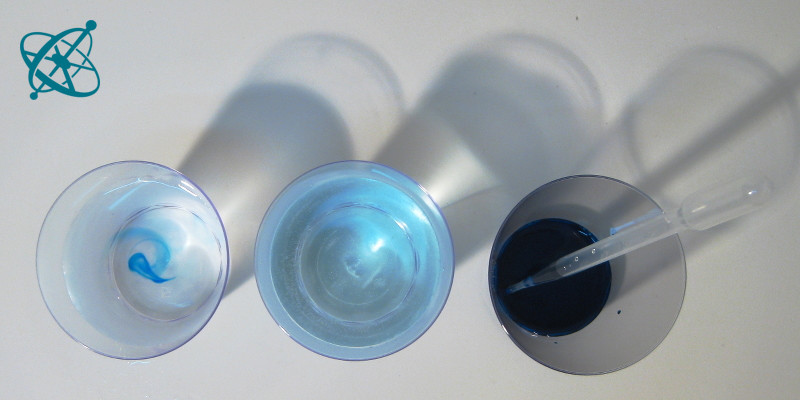
Yes, by the way they push around ink.
Heat 'n motion
With a bit of food coloring or a tea bag in hot and cold water you can quickly demonstrate the kinetic theory of temperature. Why not turn the tables and ask your students to prove (or disprove) the theory to you? Let them develop the experiment and explain to you how it provides evidence.
Molecules in hot liquids and gases move faster than in cold ones.
Critically interpret experimental results to find scientific evidence.
Hot and cold water
Food coloring (or tea bags)
After having introduced the kinetic theory of gases and liquids, challenge your students to develop an experiment to test this theory. Place glasses, hot and cold water and tea bags or food coloring on the teacher table.
Before conducting any experiment, make your students explain how exactly they plan to conduct the experiment and how it will provide useful evidence. This activity is less about the actual experiment than about the rational your students develop to proof or disproof a theory with it.
1. Using the material on the teacher table, can you prove or disprove that molecules in hot liquids move faster than in cold fluids?
If you add food coloring (or a tea bag) to water, how does the color become dispersed in the liquid?
› The water molecules bounce into the dye molecules and scatter them through the liquid.
According to kinetic theory, are the molecules moving faster in warm or in cold water?
› In warm water.
Will faster or slower water molecules distribute the dye more quickly?
› Faster molecules, i.e. warm water.
There are many possibilities to test this theory, yet with the material you placed on your table you already provided a hint. One option is to fill two water glasses with hot and cold water, respectively, and then add the teabag or a drop of food coloring. As the molecules in the hot water move faster, they collide more often with the dye molecules and distribute them quicker.



