www.sciensation.org | Ciênsação hands-on experiments are published as Open Educational resources under a Creative Commons Attribution-ShareAlike 4.0 International License.
www.sciensation.org | Ciênsação hands-on experiments are published as Open Educational resources under a Creative Commons Attribution-ShareAlike 4.0 International License.
What makes water float on water?

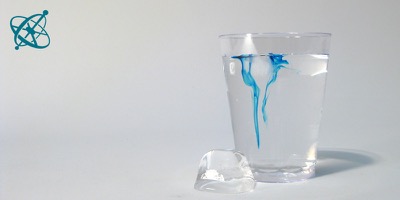
A drop of food coloring…

…beautifully reveals how the cold water…

…melted from the ice cube…

…sinks to the bottom, where it displaces…

…the warmer water that was there.

Water floating and sinking in water
Common water is among the most interesting materials on Earth. One of its many extraordinary features is the unusual dependency of its density on temperature, as seen in this simple yet beautiful experiment.
In contrast to most other materials, water has a lower density in the solid phase (as ice) than in the liquid phase.
The density of cold water is higher than that of warm water.
Observing the principle of convective heat transfer in action.
Water
Ice cubes
Food coloring
Fill a plastic cup with water. Wait a moment to let it calm down before gently adding a single ice cube. Observe without shaking or touching the water.
1. Since ice is just frozen water, how come that water can swim on water?
Ask your teacher to drop food coloring on each ice cube.
2. Why does the colored water sink?
When do things float in water?
› When their density is lower than that of water.
When do things sink in water?
› When their density is larger than that of water.
Does the density of a material depend on its temperature?
› Yes.
Does the food coloring sink if you drop it in water without an ice cube?
› No (see pictures in 'Heat 'n motion').
When the cold water sinks to the bottom, where does the warm water at the bottom go?
› It is pushed up, initiating what is called a heat convection stream.
Unlike most other materials, the density of water decreases by about 9% when freezing (going from the liquid to the solid phase). Ice is therefore less dense than water at room temperature and floats.
However, cold liquid water is denser than warm water (the maximal density is reached at +4˚C). Cold water melted from the ice cube therefore sinks in water at room temperature, as the food coloring makes visible. Warmer water from the bottom is thereby pushed up, forming a convection stream.
To be accurate, it should be mentioned that only crystalline ice has a lower density than liquid water. If water freezes extremely quickly or under high pressure, it forms amorphous solids (like glass) which may have higher densities.