www.sciensation.org | Ciênsação hands-on experiments are published as Open Educational resources under a Creative Commons Attribution-ShareAlike 4.0 International License.
www.sciensation.org | Ciênsação hands-on experiments are published as Open Educational resources under a Creative Commons Attribution-ShareAlike 4.0 International License.
How to turn a straw into a 'magic' wand that bends water?

Just give it some negative electric charge.

But careful, once you touch the water,…

…the magic literally goes down the drain!
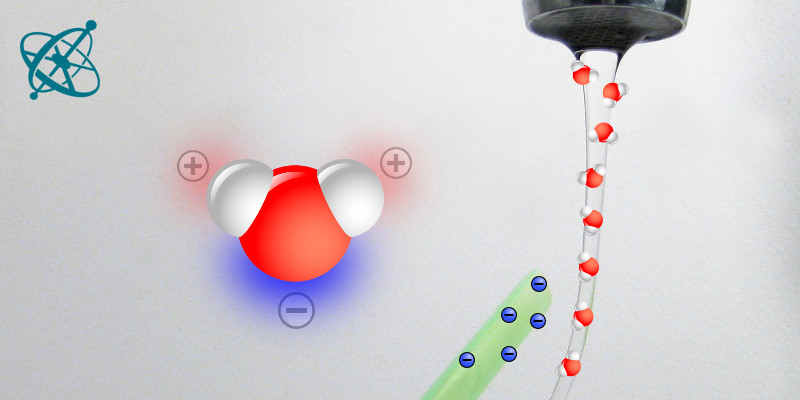
It is the polarity within each water molecule that attracts the flow to the straw.
Bending water
Discussing the implications of this well-known experiment can be of great help to 'grasp' the idea of a dipole moment in water molecules.
Ask your students to explain in a drawing why water is attracted by electrical charges. This allows them to apply what they have learned, and you to get a clearer 'picture' of their understanding.
Water has a molecular dipole moment and is therefore attracted to electrostatic charges.
Using a graphical approach to analyze natural phenomena.
Straw
Hair and paper
Two or three students can conduct this experiment simultaneously at one sink.
Alternatively, you may give this activity as homework and discuss the drawings in the next lesson.
Open a water tap just a bit, so that a fine, continuous flow comes out, but not single droplets. Then take a piece of dry paper and press with it a plastic straw against your hair. Swipe the straw between the paper and your hair about 4 times in the same direction before holding it about one centimeter from the water flow.
1. What do you see, and why does the effect disappear if you move the straw through the water?
2. Make a drawing that explains the phenomena you observed.
Does the water have an electrical charge?
› No, it is practically neutral. While there are always some ions in tap water, their respective charges cancel each other out.
Do water molecules have an electric charge?
› No, but the hydrogens' electrons 'spend more time' with the oxygen atom than with the hydrogen (oxygen atoms are 16 times heavier than hydrogen), thus causing a polarity within the molecule.
Does the electric attraction between the water and the straw also occur between water molecules?
› Yes, these are so-called hydrogen bonds.
Is water a good conductor of electricity?
› It is not a very good conductor. But when water comes in contact with the straw it can easily discharge it.
The partial positive charge of the hydrogen atoms is attracted to the negative charge of the straw. The water molecules therefore align and the flow bends towards the straw. However, once the straw touches the water, the electrons discharge to the water and the electrostatic effect disappears. A drawing explaining this phenomena could look something like the last picture of the gallery above.